sulfuric acid
Sulfuric acid is the most widely used corrosive in the world and is generally considered to be the most important industrial chemical. The corrosivity of sulfuric acid on steel and nickel alloys can vary significantly. Depending on acid concentration and the metal involved, sulfuric acid can behave as either a reducing acid or an oxidizing acid. In lower concentrations (up to about 70%) and temperatures on steel or stainless steel, it is reducing. At concentrations over 70%, and into the oleum range, the acid is oxidizing to steel and stainless steel. The best evidence of this transition is shown in the corrosion rate of Alloy 400 (Monel©) at 60° C with or without aeration (Figure 1). Note that the presence of oxygen (aeration) significantly increases the corrosion rate of Alloy 400.
Bulk sulfuric acid is normally transported and stored in concentrations which can be readily handled by carbon steel. Concentrations from 88% to 99.5% with velocities below 2 feet/second and temperatures below 120° F will leave carbon steel virtually unattacked. These conditions also describe the parameters associated with many storage tanks.
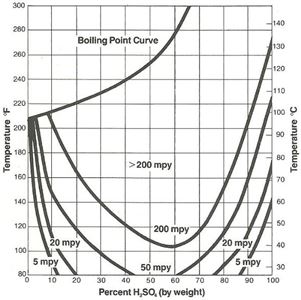
The protective iron sulfate film which forms on steel is easily damaged with increasing velocity, and 316L stainless with a more adherent protective film is often used as transfer piping for acid at the higher concentrations. However, except for a small region of resistance at the low concentration range, 316L does not offer any significant advantage over plain carbon steel other than the velocity considerations already noted. As Figure 2 illustrates, concentrations of sulfuric acid below about 85% at room temperature can result in rates in 316L in excess of 5 mils per year (mpy). Plain steel, 400 series stainless and even 304L have no practical use in this concentration range.
Concentrated sulfuric acid is hygroscopic and will absorb moisture until its concentration is reduced to about 45%. Storage tanks need to be properly vented or desiccated to avoid allowing moisture from the atmosphere into the tank. Without such precautions the concentration of acid in the vapor space will be much more corrosive than the bulk liquid. For more information on storage tank construction and handling of sulfuric acid, see the NACE Recommended Practice RP-0391.
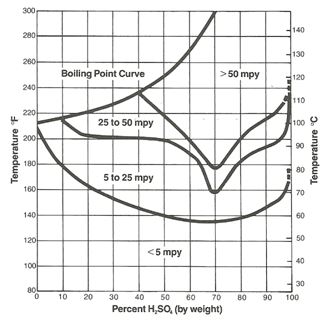
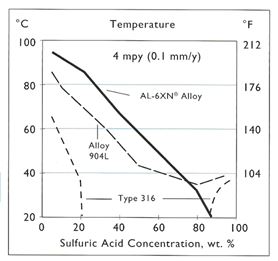
While the storage of concentrated acid can be handled effectively with low alloy steels, most sulfuric acid is usually encountered in diluted concentrations. Dilution of concentrated sulfuric is intentional for many chemical processes or in water treatment. The dilution of sulfuric acid is exothermic, but with or without this additional heat, the lower concentration of sulfuric acid will result in conditions unsuitable for mild steel or 316L. Under such conditions, materials such as Alloy 20 can offer good resistance over a wide range of acid concentrations. Figure 3 illustrates the typical resistance of Alloy 20 in sulfuric acid which offers a corrosion rate less than 5 mpy over the entire concentration range up to about 135° F. Alloys such as 904L and AL-6XN© Alloy also offer good resistance to sulfuric acid up to about 70%, but do not perform as well in the 70 – 90% range. (Figure 4) However, these alloys perform better than Alloy 20 when the acid is contaminated with chlorides above about the 5000 ppm level.
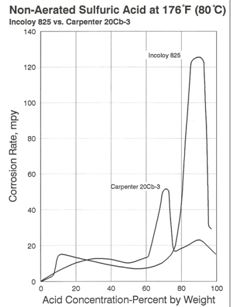
Alloy 825 is also useful in sulfuric acid over a broad range of concentrations and conditions. As shown in Figure 5, 825 performs better than Alloy 20 in 70% acid at elevated temperature, but exhibits a much higher rate of attack at concentrations of 80 – 95% acid.
Duplex alloy 2205 provides useful resistance in mild concentrations and temperatures of sulfuric acid. It offers a wider range of resistance than 316L stainless. However, small increases in temperature or acid concentration can lead to more rapid increases in corrosion rates than are seen in 316L. This can be an important consideration in start-up or upset conditions.
Lean duplex alloys have no practical use in sulfuric acid concentrations beyond the trace level. A superduplex alloy like Zeron 100 also broadens usage beyond the common duplex 2205 in the lower concentration (reducing) range. Up to about 70% acid, it is comparable to Alloy 20 in resistance at temperatures below 60° C. The resistance of Zeron 100 to sulfuric acid again gains an advantage over Alloy 20 at higher acid concentrations over 90%. It is important to recognize that as the temperature increases, the corrosion rate of the superduplex grades will rise much more rapidly than that of the austenitic alloys, such as Alloy 20.

The graphs and discussion here offer some general guidance on the use of stainless and nickel alloys in sulfuric acid. Additional tabular data can be found elsewhere on the Rolled Alloys website and various other published resources such as NACE International publication on the Corrosion Data Survey. Most published data in sulfuric and other acids is based on reagent grade acids. For most stainless and nickel alloys (not Ni-Cu alloys) industrial grade acids that contain metallic ions and salts are less aggressive than pure acid. Acid composition and other variables, such as mixed acids, can significantly change results from those initially expected. Testing of coupons also offers an approach to obtaining information specific to a unique environment. Always consider consulting a corrosion expert.


